6 Longitudinal psycho-physical co-development
Adapted from:
Longitudinal co-development of depressive symptoms and cardio-metabolic risk factors from childhood to young adulthood
Abstract
Background: Depressive symptoms and cardio-metabolic risk factors often co-occur. However, our understanding of the potential mechanisms and temporal dynamics underlying their co-development remains elusive.
Methods: This population-based cohort study examined bidirectional longitudinal associations between depressive symptoms and cardio-metabolic risk factors from age 10 to 25 years, using prospective data from the ALSPAC Study. Participants with at least one (of six) follow-up measurement for each outcome were included in the analyses. We measured depressive symptoms through self- as well as parent-reports, and assessed several cardio-metabolic risk factors (including adiposity measures, lipid profiles and inflammation).
Results: Among our 7970 (47% male, 96% White) participants, we found bidirectional, within-person associations between self-reported depressive symptoms and adiposity (i.e., fat/lean mass index, but not body mass index), across the study period. Adiposity was more stable over time (β [range] = 0.75 [0.54; 0.84]), compared to depressive symptoms (0.26 [0.12; 0.38]), and it had a stronger prospective (i.e., cross-lagged) association with future depressive symptoms (0.07 [0.03, 0.13]) compared to that between depressive symptom and future adiposity (0.04 [0.03, 0.06]). The magnitude of these associations reached its peak between 14 and 16 years. We did not find evidence of cross-lagged associations in either direction between depressive symptoms and waist circumference, insulin, triglycerides, LDL cholesterol or C-reactive protein.
Conclusions: These findings suggest a bidirectional relationship between depressive symptoms and cardio-metabolic risk factors, particularly adiposity (i.e., fat/lean mass). Adiposity showed a stronger prospective association with future depressive symptoms, than vice versa, however their relationship revealed more reciprocal than previously thought.
Links
Keywords
Depressive symptoms; Cardio-metabolic risk; Comorbidity; Longitudinal; ALSPAC.
Abbreviations
Avon Longitudinal Study of Adults and Children (ALSPAC), Autoregressive Latent Trajectory Model with Structured Residuals (ALT-SR), Body Mass Index (BMI), C-reactive protein (CRP), Fat Mass Index (FMI), High- / Low-Density Lipoprotein (HDL / LDL [cholesterol]), Lean Mass Index (LMI), Random-Intercept Cross-Lag Panel Model (RI-CLPM), Short Mood and Feelings Questionnaire (SMFQ).
6.1 Introduction
Over 20% of the general population faces at least one depressive episode in their lifetime (Gutiérrez-Rojas et al., 2020) and a growing number of adolescents experience depressive symptoms before the age of 20 years (Keeley, 2021; Patalay & Gage, 2019). Concurrently, the prevalence of child obesity and related cardio-metabolic risk factors is alarmingly high, affecting one in three children and almost half of young adults (NCD-RisC, 2017; WHO, 2022). Moreover, depressive symptoms and cardio-metabolic risk factors often co-occur (Anwar et al., 2018; Blasco et al., 2020; Gutiérrez-Rojas et al., 2020). For example, a large meta-analysis showed that people suffering from depression had a 58% higher risk of developing obesity, while individuals with obesity had a 55% elevated risk of developing depression, compared to the general population (Luppino et al., 2010).
Several potential mechanisms have been proposed to explain this observed comorbidity, both in adults (Carey et al., 2014; Milaneschi et al., 2019) and children (Defina et al., 2023; Sutaria et al., 2019). However, scientific efforts to empirically model the co-developmental processes that may underlie this comorbidity (i.e., temporal precedence and/or bidirectional relationships) have been sparse, inconsistent, and mostly focused on adult or aging populations (Forman-Hoffman et al., 2007; Konttinen et al., 2014) and/or genetic liabilities (Chen et al., 2023; Jokela & Laakasuo, 2023).
In the pediatric literature, a small number of studies have investigated longitudinal relationships between body mass index (BMI) and internalizing / emotional problems (an early marker of depressive symptoms) (Bradley et al., 2008; Jansen et al., 2013; Patalay & Hardman, 2019; Zhou et al., 2022). The majority of these studies found higher BMI to precede increases in internalizing symptoms, but not the other way around (Bradley et al., 2008; Jansen et al., 2013; Patalay & Hardman, 2019), in line with some (but not all, e.g. Chen et al. (2023)) Mendelian randomization studies investigating the causal effect of obesity on depression (Jokela & Laakasuo, 2023). A more recent investigation however, did identify reciprocal relationships between BMI and internalizing symptoms, by employing more advanced modeling frameworks, capable of decomposing between- and within-person variances over time (Zhou et al., 2022).
Importantly, while changes in fat mass are hypothesized to be a key mechanism in these studies, they rely exclusively on BMI measures, which cannot discriminate between fat mass and lean (e.g. muscle) mass, and are thus a suboptimal measure of cardio-metabolic risk (Dencker et al., 2007; Liu et al., 2013; Vanderwall et al., 2017). Moreover, existing evidence largely relies on parental reports of depressive symptoms, which may be less sensitive compared to self-reports (Cohen et al., 2019). Finally, these studies only investigated relatively short follow-up periods and early developmental windows (i.e., 1 to 5 years, from childhood to early adolescence), leaving the period between adolescence to young adulthood, which is when these conditions typically find their onset, largely unexplored.
To address these gaps, we aimed to characterize the temporal dynamics underlying the (co-)development of depressive symptoms and cardio-metabolic risk as they unfold jointly across 15 years, from the age of 10 to 25 years. We investigated several cardio-metabolic risk factors (including total fat and lean mass) and multi-informant reports on depressive symptoms.
In the spirit of open science, we also provide an open-source interactive web-application that can be used, alongside this article, to flexibly explore our results and verify their robustness across multiple outcomes and analytical choices.
6.2 Methods
Sample and measures
This study is based on data from the Avon Longitudinal Study of Parents and Children (ALSPAC). Pregnant women resident in Avon (UK) with expected delivery dates between 1st April 1991 and 31st December 1992 were invited to take part in the study. The initial number of pregnancies enrolled was 14,541, with 13,988 children who were alive at 1 year of age. When children were approximately 7 years old, additional eligible cases were re-invited, resulting in a total sample of 15,447 pregnancies and 14,901 children who were alive at 1 year of age (Boyd et al., 2012; Fraser et al., 2013; Northstone et al., 2019). Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Bristol (Harris et al., 2009). Please note that the ALSPAC website contains details of all the data that is available through a fully searchable data dictionary and variable search tool (http://www.bristol.ac.uk/alspac/researchers/our-data/).
Ethical considerations
Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees. Informed consent was obtained from participants’ parents.
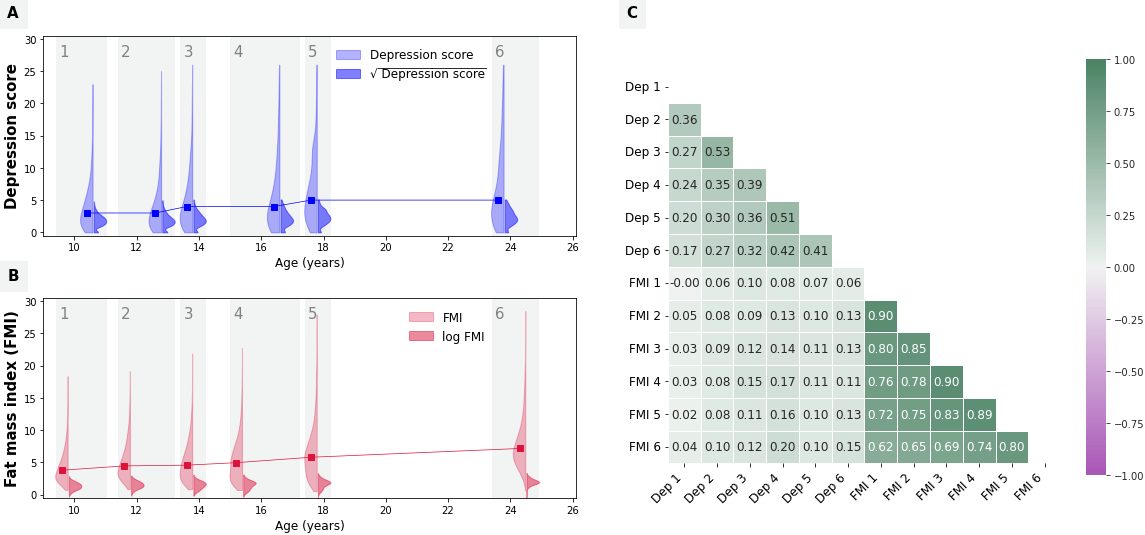
Depressive symptoms
Depressive symptoms were repeatedly measured using the Short Mood and Feelings Questionnaire (SMFQ) (Angold et al., 1995; Kwong, 2019). The instrument includes 13 items referred to the past two weeks and scored between 0–2 (i.e., “not true” / “sometimes true” / “true”). A summary score ranging between 0–26 was computed at each occasion, with higher scores indicating greater depressive symptoms (Figure 6.1 A). The SMFQ was administered to the child/young person on six occasions between the ages of 10 and 25 years (at the median ages of 10.6, 12.8, 13.8, 16.6, 17.8, and 23.8 years). The questionnaire was also completed by participants’ parents (most commonly mothers) on four additional occasions (when children were on average 9.6, 11.7, 31.1, and 16.7 years old) which were used in secondary analyses.
Cardio-metabolic risk markers
The primary cardio-metabolic measure examined in this study was fat mass index (FMI; Figure 6.1 B), computed as participants’ total body fat mass divided by their squared height (kg/m2). Total body fat mass was derived from whole body dual energy X-ray absorptiometry (DXA) scans at six occasions (at median ages of 9.8, 11.8, 13.8, 15.4, 17.8, and 24.5 years) (Dangardt et al., 2019).
Ten other cardio-metabolic risk factors were further examined in secondary analyses, including lean mass index (LMI), body mass index (BMI), waist circumference, android fat mass, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol levels, triglycerides, insulin, and C-reactive protein (CRP) (see Appendix S1, Figure S1.
Sample demographics
Participant sex was measured at birth. Participants’ parents further reported on their ethnical identity (at recruitment) and their educational attainment (when children were 5 years old).
Statistical analyses
Analyses were conducted using R version 4.2.2 (R Core Team, 2021); model specification and fit was implemented in lavaan (version 0.6–16) (Rosseel, 2012). All scripts are publicly available here.
Data pre-processing
To ensure optimal model conversion, we first performed data cleaning by setting extreme outlier values (i.e., > 5 interquartile ranges above the third quartile or below the first quartile) to missing. We then transformed the data to reduce skewness, using a square root transformation (for depressive symptoms scores; see Figure 6.1 A) or a ln transformation (for cardio-metabolic risk markers; see Figure 6.1 B). Finally, we performed min-max normalization (see Equation 6.1) to rescale of all variables to a [0, 1] range. \[ x_{norm} = \frac{x - \min(x)}{\max(x) - \min(x)} \tag{6.1}\]
This procedure allowed us to obtain comparable estimates across different units and improve model convergence, while preserving relative differences within each variable over time (e.g. mean variations).
Main analyses
We fit a lag-1 random-intercept cross-lag panel model (RI-CLPM) (Hamaker et al., 2015) to characterize the relationship between self-reported depressive symptoms and fat mass index across 6 time points (~10, 12, 14, 16, 18 and 24 years). The model was specified as a structural equation model composed of four parts (see also Appendix S2
- A between-person part, consisting of the “random intercepts” (\(η_{\ dep}\) and \(η_{\ FMI}\)). These are latent variables that have each measurement occasion as indicator and factor loadings set to 1. They reflect stable (i.e., “time-invariant”) between-person differences (e.g., some children may have systematically higher fat mass over time compared to others).
- A within-person part, consisting of “within-unit fluctuations”: time-specific residual terms specified as latent variables with factor loading set to 1, and (measurement error) variances set to 0. They represent random changes that make observations unique, allowing individuals to differ (from themselves) at each occasion. For example, these could reflect a life event that raises/lowers a person’s depression at a given time \(t\).
- The (lag-1) regressions between the within-unit components: i.e. the auto-regressive and cross-lagged terms.
- auto-regressive terms quantify the persistence (or “inertia”) of a construct, i.e. its tendency to retain its state over time. For example, AR~* dep*~ captures the proportion of past depression that persists directly to the next measurement occasion.
- In contrast, cross-lagged relations measure the proportion of past variance in one variable that is reflected in the other variable at the next measurement occasion, and are therefore used to infer (Granger) causality. For example, CL~* dep*~ indicates how much within-person variance in depression at time \(t\) is uniquely explained by FMI at time point \(t\) – 1 (controlling for the persistence of past values of depression).
- Covariances in the between- and within- person part.
- To control for between-person trends that may confound the (within-person) system dynamics reflected by auto-regressive and cross-lagged terms, the covariance between \(η_{\ dep}\) and \(η_{\ FMI}\) is freely estimated.
- Similarly, because within-unit fluctuations may be non-independent (e.g., when a random change affects both variables simultaneously) this is modelled by estimating their covariance within each wave.
Full information maximum likelihood (FIML) estimation was used to account for missing patterns that may not conform to MCAR. Coefficients were standardized and conventional robust standard errors were used to compute 95% confidence intervals (95%CI).
Model fit was evaluated using the Root Mean Square Error of Approximation (RMSEA), the Comparative Fit Index (CFI), the Tucker Lewis Index (TLI), and the Standardized Root Mean Square Residual (SRMR). We considered model fit to be adequate when: RMSEA ≤ 0.05, CFI and TLI ≥ 0.95, and SRMR < 0.08 (Kline, 2016).
Exploratory analyses
We further conducted three sets of exploratory follow-up analyses.
- We replaced FMI with each of 10 alternative cardio-metabolic risk markers, including measures of adiposity (i.e., BMI, LMI, android fat mass and waist circumference), lipid profiles (i.e., HDL and LDL cholesterol levels, and triglycerides), insulin levels and inflammation (i.e., CRP).
- We replaced self-reported depressive symptoms scores with maternal reports (at the available time points).
- Estimated an Autoregressive Latent Trajectory Model with Structured Residuals (ALT-SR) with linear latent growth as an alternative to the RI-CLPM. Briefly, the ALT-SR is a more flexible modelling approach, which allows to explicitly model developmental trends (i.e. latent random slopes), in addition to the stable trait-like between-person differences (i.e., latent random intercepts) (Curran et al., 2014).
Note that the project web-application offers the opportunity for researchers to interact with model settings and examine the robustness of findings against violations of modelling assumptions, such as the temporal stability of the between- and within-person components (Appendix S2).
6.3 Results
Descriptive statistics
Sample descriptives are presented in Table 6.1 and Figure 6.1. The main analytical sample consisted of 7970 (47% male) participants, who had at least one measurement of depressive symptoms and FMI; 96% of participants’ parents identified as ethnically “White”.
Both depressive symptoms and FMI increased slightly with age (see Figure 6.1 A-B); their cross-sectional correlations ranged from 0.00 to 0.17 (r mean = 0.10; Figure 6.1 C).
Girls had systematically higher FMI compared to boys across time points, and they reported higher depressive symptoms scores from the ages of 14 years onwards.
(A, B) The distribution of observed values for SMFQ depressive scores (A) and fat mass index (B) is presented on the y-axis against measurement time (x-axis). In the violin plots, lighter colors are used to represent the original value distributions, while darker colors represent the same variable distributions and after data transformation was applied (i.e., square root for depressive symptoms scores and log transformation for FMI). The line graph connects the median points (in the original data scale) at each timepoint. (C) The univariate, pairwise Pearson correlation coefficients between each repeated measure of depressive symptoms (Dep) and fat mass index (FMI).
| Sample descriptives ALSPAC cohort |
|||
|---|---|---|---|
| Total sample (n=7970) |
Male participants (n=3769) |
Female participants (n=4185) |
|
| SMFQ depressive symptom score, median (range) [% missing values] | |||
| 10 years | 3 (0-23) [15%] | 3 (0-23) [14%] | 3 (0-21) [17%] |
| 12 years | 3 (0-25) [19%] | 3 (0-25) [17%] | 3 (0-24) [21%] |
| 14 years | 4 (0-26) [24%] | 3 (0-26) [21%] | 4 (0-26) [26%] |
| 16 years | 4 (0-26) [43%] | 3 (0-26) [51%] | 5 (0-26) [36%] |
| 18 years | 5 (0-26) [45%] | 4 (0-26) [51%] | 6 (0-26) [40%] |
| 24 years | 5 (0-26) [54%] | 4 (0-26) [66%] | 5 (0-26) [42%] |
| Fat mass index, median (range) [% missing values] | |||
| 10 years | 3.8 (0.7-18.4) [50%] | 3.0 (0.7-15.4) [49%] | 4.4 (1.0-18.4) [51%] |
| 12 years | 4.4 (0.9-19.1) [51%] | 3.8 (0.9-16.8) [50%] | 5.0 (1.1-19.1) [51%] |
| 14 years | 4.6 (0.8-21.9) [24%] | 3.1 (0.8-17.7) [22%] | 5.7 (1.5-21.9) [27%] |
| 16 years | 5.0 (0.7-22.8) [36%] | 2.8 (0.7-20.0) [36%] | 6.4 (1.5-22.8) [36%] |
| 18 years | 5.8 (0.5-27.9) [40%] | 3.4 (0.5-24.1) [44%] | 7.1 (0.5-27.9) [36%] |
| 24 years | 7.2 (0.6-28.5) [53%] | 5.7 (1.8-23.2) [63%] | 8.0 (0.6-28.5) [44%] |
| Sex, n (%) | |||
| Male | 3769 (47%) | ||
| Female | 4185 (53%) | ||
| Ethnic background, n (%) a | |||
| Non-white | 280 (4%) | 134 (4%) | 146 (4%) |
| White | 6718 (96%) | 3215 (96%) | 3503 (96%) |
| Maternal education, n (%) b | |||
| No education | 210 (3%) | 89 (3%) | 121 (4%) |
| Medium | 4836 (78%) | 2374 (78%) | 2462 (77%) |
| High | 1164 (19%) | 561 (19%) | 603 (19%) |
| Paternal education, n (%) b | |||
| No education | 349 (7%) | 165 (6%) | 184 (7%) |
| Medium | 3475 (66%) | 1676 (65%) | 1799 (66%) |
| High | 1469 (28%) | 730 (28%) | 739 (27%) |
| a Ethnic backgroung: "White" if both patents identified as "White"; "Non-white" if either parent identified as "Black Caribbean", "Black African", "Other black", "Indian", "Pakistani", "Bangladeshi", "Chinese", or "Other". |
|||
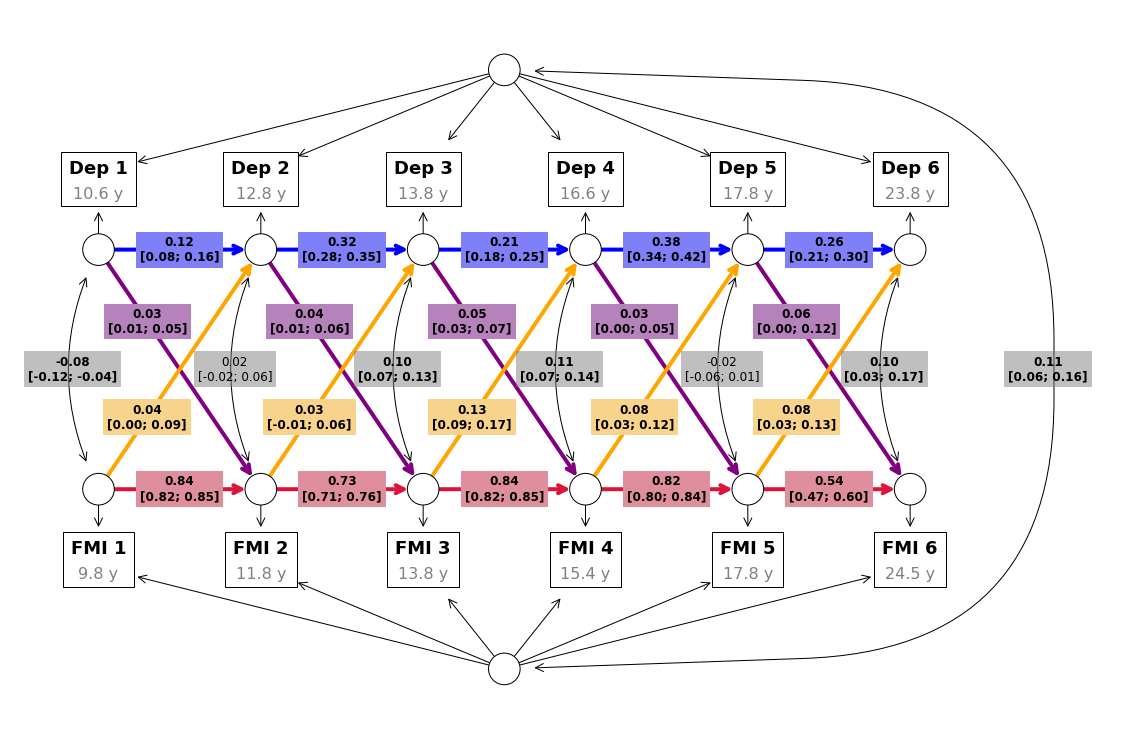
Main results
Results of the main analyses, examining the co-development of depressive symptoms and FMI, are summarized in Table 6.2 and Figure 6.2. The model showed good fit (\(χ^2\)(37) = 356.77, p<0.001; RMSEA [95%CI] = 0.033 [0.030-0.036]; CFI = 0.991; TLI = 0.983; SRMR = 0.027).
We found positive auto-regressive associations, indicating substantial within-person stability over time in FMI (mean \(β\) [range] = 0.75 [0.54; 0.84], SE = 0.016) and, to a lower extent, in depressive symptoms (\(β\) [range] = 0.26 [0.12; 0.38], SE = 0.022).
After accounting for within-person (i.e., autoregressive associations) and between-person stability (i.e., random intercepts), the following within-person cross-lag dynamics emerged from our models (Table 6.2): higher FMI was associated with increased subsequent depressive symptoms across the study period, except between 12 and 14 years (\(β\) [range] = 0.07 [0.03, 0.13], SE = 0.030); higher depressive symptoms were associated with increased subsequent FMI, although these associations were weaker on average, compared to those between FMI and future depressive symptoms (\(β\) [range] = 0.04 [0.03, 0.06], SE = 0.01).
| Auto-regressive and cross-lag associations between depressive symptoms and FMI |
||||||
|---|---|---|---|---|---|---|
| Term | Estimate [95%CI] |
Median age (years) |
Time lag (years) |
Yearly estimate [95%CI] |
||
| Auto-regressive associations | Fat mass index (FMI) | 1 | 0.84 [0.82; 0.85] | 9.8 → 11.8 | 2.0 | 0.42 [0.41; 0.43] |
| 2 | 0.73 [0.71; 0.76] | 11.8 → 13.8 | 2.0 | 0.37 [0.36; 0.38] | ||
| 3 | 0.84 [0.82; 0.85] | 13.8 → 15.4 | 1.6 | 0.52 [0.51; 0.53] | ||
| 4 | 0.82 [0.80; 0.84] | 15.4 → 17.8 | 2.4 | 0.34 [0.33; 0.35] | ||
| 5 | 0.54 [0.47; 0.60] | 17.8 → 24.5 | 6.7 | 0.08 [0.07; 0.09] | ||
| Depressive symptoms | 1 | 0.12 [0.08; 0.16] | 10.6 → 12.8 | 2.2 | 0.05 [0.04; 0.07] | |
| 2 | 0.32 [0.28; 0.35] | 12.8 → 13.8 | 1.0 | 0.32 [0.28; 0.35] | ||
| 3 | 0.21 [0.18; 0.25] | 13.8 → 16.6 | 2.8 | 0.08 [0.06; 0.09] | ||
| 4 | 0.38 [0.34; 0.42] | 16.6 → 17.8 | 1.2 | 0.31 [0.28; 0.35] | ||
| 5 | 0.26 [0.21; 0.30] | 17.8 → 23.8 | 6.0 | 0.04 [0.03; 0.05] | ||
| Cross-lag associations | FMI→Dep | 1 | 0.04 [0.00; 0.09] | 9.8 → 12.8 | 3.0 | 0.01 [0.00; 0.03] |
| 2 | 0.03 [-0.01; 0.06] | 11.8 → 13.8 | 2.0 | 0.01 [-0.01; 0.03] | ||
| 3 | 0.13 [0.09; 0.17] | 13.8 → 16.6 | 2.8 | 0.05 [0.03; 0.06] | ||
| 4 | 0.08 [0.03; 0.12] | 15.4 → 17.8 | 2.4 | 0.03 [0.01; 0.05] | ||
| 5 | 0.08 [0.03; 0.13] | 17.8 → 23.8 | 6.0 | 0.01 [0.01; 0.02] | ||
| Dep→FMI | 1 | 0.03 [0.01; 0.05] | 10.6 → 11.8 | 1.2 | 0.03 [0.01; 0.05] | |
| 2 | 0.04 [0.01; 0.06] | 12.8 → 13.8 | 1.0 | 0.04 [0.01; 0.06] | ||
| 3 | 0.05 [0.03; 0.07] | 13.8 → 15.4 | 1.6 | 0.03 [0.02; 0.04] | ||
| 4 | 0.03 [0.00; 0.05] | 16.6 → 17.8 | 1.2 | 0.02 [0.00; 0.04] | ||
| 5 | 0.06 [0.00; 0.12] | 17.8 → 24.5 | 6.7 | 0.01 [0.00; 0.02] | ||
| Cross-sectional correlations | FMI ~ Dep | 1 | -0.08 [-0.12; -0.04] | 9.8 ~ 10.6 | 0.8 | |
| 2 | 0.02 [-0.02; 0.06] | 11.8 ~ 12.8 | 1.0 | |||
| 3 | 0.10 [0.07; 0.13] | 13.8 ~ 13.8 | 0.0 | |||
| 4 | 0.11 [0.07; 0.14] | 15.4 ~ 16.6 | 1.2 | |||
| 5 | -0.02 [-0.06; 0.01] | 17.8 ~ 17.8 | 0.0 | |||
| 6 | 0.10 [0.03; 0.17] | 23.8 ~ 24.5 | 0.7 | |||
Results of the main analysis (i.e., beta values and their 95% confidence intervals) are displayed within their implied directed graph. Autoregressive associations are shown in red for FMI and in blue for depressive symptoms (Dep), while cross-lag effects are shown in yellow for FMI to depressive symptoms paths and in purple for depressive symptoms to FMI paths. Grey boxed enclose the correlation coefficients (and their 95% confidence intervals) between the two random intercepts, as well as the cross-sectional correlations between the two outcomes at each measurement wave.
We additionally found a positive correlation between the random intercepts (\(r\) [95%CI] = 0.11 [0.06-0.16]), suggesting some stability of between-person associations between depressive symptoms and FMI.
Exploratory analysis results
Please visit the project web-application for an interactive report of all results obtained from exploratory analyses. We highlight and summarize below a few key findings.
Other cardio-metabolic risk factors
The pattern of reciprocal cross-lag paths identified in the main analysis was remarkably consistent when lean mass (rather than fat mass) index was examined as a cardio-metabolic risk factor (average standardized \(β\) for LMI to depressive symptoms = -0.08, SE = 0.029; depressive symptoms to LMI = -0.04, SE = 0.007). When BMI was included in the model instead, only the prospective association between BMI and depressive symptoms remained (\(β\) = 0.05, SE = 0.035), while depressive symptoms did not seem to affect future BMI (\(β\) < 0.01, SE = 0.008). Android fat mass and depressive symptoms showed reciprocal associations only between 14 and 16 years (android fat to depressive symptoms = 0.14 [0.06; 0.21]; depressive symptoms to android fat = 0.04 [0.01; 0.07]).
Surprisingly, a positive within-person reciprocal association between depressive symptoms and HDL (but not LDL) cholesterol levels was detected, between 16 and 25 years (see Figure 6.3). In contrast, the correlation between the random intercepts of depressive symptoms and HDL cholesterol was negative (-0.18 [-0.27; -0.09]). We did not find evidence of direct associations in either direction when waist circumference, insulin, triglycerides, or CRP were considered in relation to self-reported depressive symptoms; however, a stable between-person association was detected for waist circumference (0.10 [0.04; 0.15]), insulin (0.12 [0.01; 0.23]) and CRP (0.11 [0.01; 0.20]).
Maternal reports of depressive symptoms
The reported findings were relatively consistent (albeit weaker) when maternal reports of depressive symptoms were used in place of self-reports (average standardized βs for FMI to depressive symptoms = 0.09, SE = 0.041; depressive symptoms to FMI = 0.04, SE = 0.013; see Figure S3). Please visit the project web-application for a complete report of these findings.
The standardised regression coefficients for the within-person cross-lag associations (and their 95% confidence intervals) are presented along the x-axis, for each alternative cardio-metabolic risk factor, listed on the y-axis. The temporal lag each estimate refers to is specified in years on the y-axis. Association estimates from cardio-metabolic risk factor to lagged depressive symptoms are presented in yellow on the left and those between depressive symptoms and lagged cardio-metabolic risk factor are shown in purple on the right. In the last column on the right of the graphs, the estimated correlation coefficient between the random intercepts of each construct (and its 95% confidence interval) is reported.
Alternative modelling approaches: the ALT-SR model
The results of exploratory models including random slopes are discussed in the supplement (Appendix S3 and Figure S4). We highlight here only a few key findings. When between-person differences in developmental (linear) trends were considered in the model, a slightly different pattern of CL relationships emerged. In particular, the CL effects of fat / lean mass on later depression no longer peaked between 13-15 years but rather kept increasing linearly over time for the duration of follow-up (see Figure S4).
6.4 Discussion
In this population-based study, we characterized the between- and within-person associations governing the co-development of depressive symptoms and cardio-metabolic risk factors, from age 10 to 25 years. Specifically, we found bidirectional, within-person associations between depressive symptoms and adiposity (i.e., fat/lean mass index, but not body mass index). Adiposity was more stable over time, compared to depressive symptoms, and it had a stronger prospective association with future depressive symptoms compared to that between depressive symptom and future adiposity.
The co-development of depressive symptoms and adiposity: fat vs. weight measures
Interestingly, the pattern of reciprocal associations between depressive symptoms and adiposity was only evident when using fat and lean mass measures, not BMI. The prospective relationship between earlier depressive symptoms and later BMI was not detectable, similar to what has been reported in previous genetic (Jokela & Laakasuo, 2023) and longitudinal epidemiological studies conducted in children (Bradley et al., 2008; Jansen et al., 2013; Patalay & Hardman, 2019).
This is important because, despite its popularity, BMI is not the best indicator of cardio-metabolic risk (e.g., a high BMI may also reflect high muscle mass, and a normal BMI does not exclude a high body fat percentage) (Liu et al., 2013). Compared to fat-based measures, BMI was also a weaker predictor of depressive symptoms (as well as other physical heath complications) in older adults (Fulton et al., 2022; Hryhorczuk et al., 2013; Milaneschi et al., 2019; Weber-Hamann et al., 2006).
Based on this evidence, we recommend the use of fat or lean mass measures when assessing the relationship between adiposity and depressive symptoms, as relying on BMI only may lead the erroneous conclusion that increased adiposity is only an antecedent (rather than a consequence) of early-onset depressive symptoms.
Maternal- vs. self-reports of depressive symptoms
We found a generally similar pattern of relationships between depressive symptoms and adiposity, when using maternal reports of depressive symptoms (rather than self-reports). However, associations were weaker when using maternal reports and they were less consistent across time points. Previous studies have relied solely on maternal reports when investigating relationships with child adiposity, which may have further contributed to inconsistencies in the reported timing, direction, and magnitude of these associations.
The wheels of a “vicious cycle”? reciprocal influences vs. stable differences
Several potential mechanisms could modulate and/or explain the bidirectional within-person dynamics highlighted in this study. For example, higher adiposity may affect psychological well-being, through reduced self-esteem, and increased feelings of shame, isolation, and body image dissatisfaction (Sjöberg et al., 2005), especially during adolescence. Adipose tissue is also an endocrine organ, involved in the production of oestrogen and pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) (Kyrou et al., 2006). These cytokines contribute to chronic low-grade systemic inflammation, which has been implicated in the development of common depressive symptoms such as anhedonia, fatigue, concentration problems and social withdrawal (Slavich & Irwin, 2014). In turn, depressive symptoms may increase the risk for subsequent adiposity through increased sedentary behavior, reduced sleep quality (Haarasilta et al., 2004), and the adoption of poorer (e.g., pro-inflammatory) dietary patterns (Lassale et al., 2019).
Interestingly, in addition to these within-person associations (which are directional and time-specific) we also found some stable between-person associations between depressive symptoms and adiposity. This is an indication that a consistent portion of the comorbidity burden can be better explained by “shared risk factors” that are largely time-invariant (e.g., sex, genetic liability, or residual confounders such as parenting practices), rather than by the reciprocal influence between constructs.
Depressive symptoms and chronic inflammation
The relationship between depressive symptoms and CRP, for instance, was better characterised in our dataset by a stable between-person association (i.e. people with higher depressive symptoms tend to also have higher CRP levels) rather than by a system of direct reciprocal influences (i.e. higher CRP leading to increased depressive symptoms at the next measurement occasion, or vice versa).
Depressive symptoms and lipid profiles
With respect to lipid profiles, a similar relationship pattern emerged between depressive symptoms and insulin levels (i.e., no reciprocal effects but rather a stable between-person association). We also detected interesting differences in the between- vs. within-person relationships between depressive symptoms and HDL cholesterol. Indeed, while the between-person correlation between random intercepts was negative (i.e., higher depressive symptoms – lower HDL cholesterol), as expected based on previous research (Penninx et al., 2013), we found positive (reciprocal) within-person associations (i.e., higher depressive symptoms were prospectively related to higher HDL cholesterol and vice versa). While similar associations have been reported before (Jia et al., 2020; Khalfan et al., 2023; Shin et al., 2008), this finding was somewhat surprising, and it should be interpreted with caution. Similarly, associations between depressive symptoms and higher LDL cholesterol and triglycerides have been reported in the literature, but rather inconsistently, and mostly in older and / or clinical populations (Ashwin et al., 2024; Khalfan et al., 2023; Shin et al., 2008).
While the biological pathways connecting depression and lipid profile abnormalities remain far from clear, it has been proposed that lower cholesterol may reduce serotonin receptor exposure, impairing mood regulation (Ashwin et al., 2024). Additionally, chronic stress and cortisol imbalances (i.e. HPA axis dysregulation) often reported in depressed individuals may alter lipid metabolism, contributing to both low and high lipid levels (Ashwin et al., 2024).
Strengths, limitations and future directions
This is one of the very few studies capable of testing bidirectional (prospective) relationships between depressive symptoms and cardio-metabolic risk factors. To the best of our knowledge, it is the first to investigate them across such an extended developmental period. We highlighted associations with a host of cardio-metabolic risk factors (including weight- and fat-based measures of adiposity, lipid profiles and inflammatory markers), and multi-informant reports of depressive symptoms. We documented both between- and within-person relationships, which were not necessarily consistent with each other (Berry & Willoughby, 2017) (as in the case of HDL cholesterol) and have separate implications for both mechanistic (i.e., causal) interpretations and clinical recommendations for prevention and/or intervention efforts. Finally, we provide an open-access web-application that allows researchers to explore and verify the robustness of our results.
However, these findings should be interpreted in light of some important limitations.
First, because the repeated measures leveraged by our models were collected every 1 to 6 years, these results are only informative about processes/dynamics that take place on this temporal scale. Higher temporal granularity, e.g., monthly assessments, may be needed to confirm, further refine or disprove these findings. For example, the smaller association between depressive symptoms and future adiposity, compared to that between adiposity and future depressive symptoms, could be due partly (or entirely) to inherent differences in the fluctuation of depressive mood vs. the persistence of adiposity over several years. Likewise, the lack of reciprocal prospective associations between depressive symptoms and the other markers of cardio-metabolic heath investigated here (including lipid profiles and systemic inflammation markers) could be explained by the transient nature of these measures. For example CRP is known to have a very short half-life (~19 hours), reflecting acute rather than chronic inflammatory processes (Mouliou, 2023).
Second, while the inclusion of random intercepts can help reduce bias, e.g., by controlling for direct and indirect confounding effects from multiple sources (Murayama & Gfrörer, 2024), we cannot completely eliminate such bias. For example, our models do not adequately handle either non-linear nor time-varying effects of time-invariant confounders, potentially failing to account for critical biological and environmental factors at play. For example, both depressive symptoms and adiposity have been linked with endocrine processes, which are particularly salient during puberty. Moreover, similar to most prior studies, the findings presented here are based on an ethnically homogeneous sample of “White” children from the UK, and may not be generalisable to other ethnic (or national) groups. Finally, depression is a very heterogeneous condition, characterized by distinct symptom clusters (e.g., vegetative symptoms such as fatigue and appetite changes, but also affective, cognitive, and somatic complaints). The current study (not unlike prior literature) relied on “global”, aggregated measures of depression, which obscure symptom-level variations, potentially hindering the mechanistic understanding of the dynamics involved in the relationship between depression and cardio-metabolic health. Future studies are therefore warranted to clarify symptom-level dynamics and confirm whether these findings are truly independent of factors such as puberty, ethnicity, lifestyle and/or socio-economic status.
Conclusions
In summary, this study addresses several important limitations of previous research, which taken together may have hindered our understanding of the relationship between depressive symptoms and cardio-metabolic risk factors over the course of development. Indeed, we show that when these constructs are measured more adequately (i.e., using self-reports of depressive symptoms and fat-based measures of adiposity), their relationship appears more reciprocal than previously thought. This is especially important in light of the increasing prevalence of both early-onset depressive symptoms and child obesity, as well as their substantial, enduring consequences for life-long health and well-being.